Informed Consent Template Irb
This page goes over.
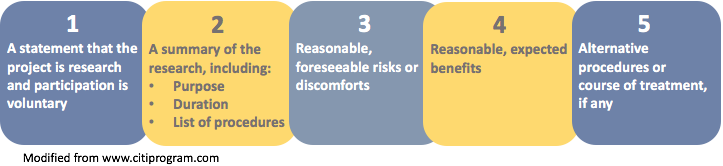
Informed consent template irb. 07 28 11 requesting waivers and exceptions to informed consent. Informed consent one of the most important considerations in research involving a human subject is the concept of informed consent. Consent templates types of informed consent. The irb must ensure that the documents and process for obtaining research informed consent satisfies adequate standards for the protection of human participants.
Strongly recommended for studies that involve the collection of biospecimens andor genetic or genomic analysis particularly federally sponsored clinical trials that are required to post a consent document on a public website. Addendum consent templates addendum consent template for non treatment studies for new procedures. The use of templates can assist the investigator in preparing the consent documents for resea. Consent templates are provided as a convenience to our researchers.
Informed consent templates 2018 common rule new irb hsbs biospecimen consent template. Obtaining and documenting informed consent v. A researcher may not involve a human subject in research that is covered by the federal guidelines unless the legally effective informed consent of the subject or the subjects legally authorized representative lar has been obtained. Consent form templates sample assent forms other forms exempt consent templates and guidance consent form templates.
Institutional biosafety committee irb institutional review board oria office of research integrity and assurance osp office of sponsored. Informed consent refers to both the process of providing participants information about the research as well as the documentation that is used to ensure that consent for participation is fully informed. Informed consent assent templates obtaining the consent of a subject is a process that goes far beyond asking for a signature on a document. A collection of informed consent.
Click here for guidance on informed consent from the office of human research protection ohrp. A collection of informed consent assent and debriefing templates that can be used for your human participant research study. Potential participants must understand the nature of the study the risks discomforts inconveniences and potential benefits involved if they are to make an informed decision. There are separate consent templates for exempt research which includes some research that involves educational tests surveys interviews or focus groups.
Irb consent form templates. If you prefer to write your own consent document you may do so but be sure to include all required elements of informed consent.