Process Validation Protocol Template For Api
Template for an example methods validation protocol 171 i.
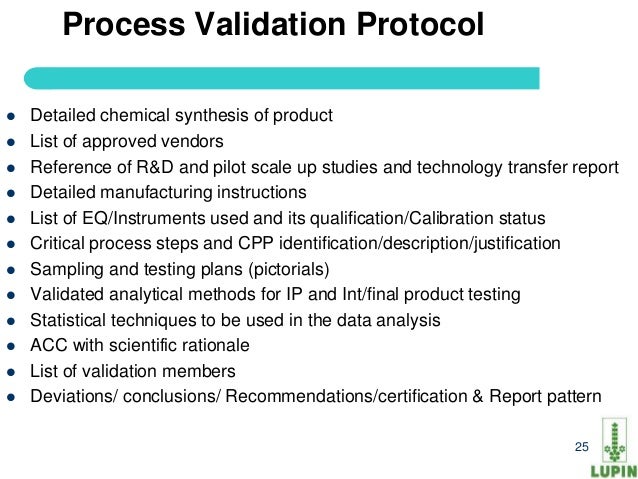
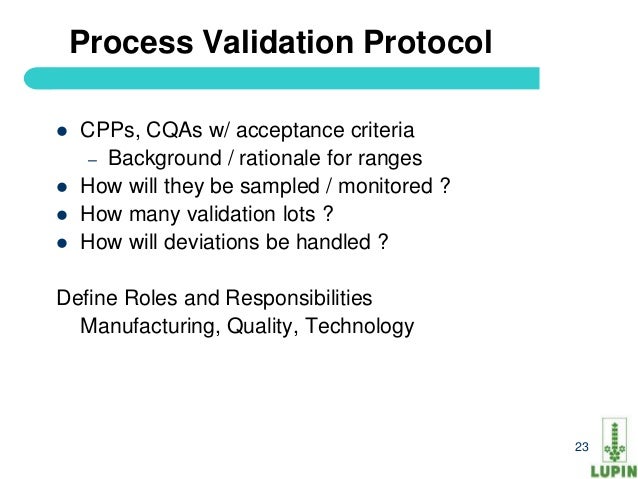
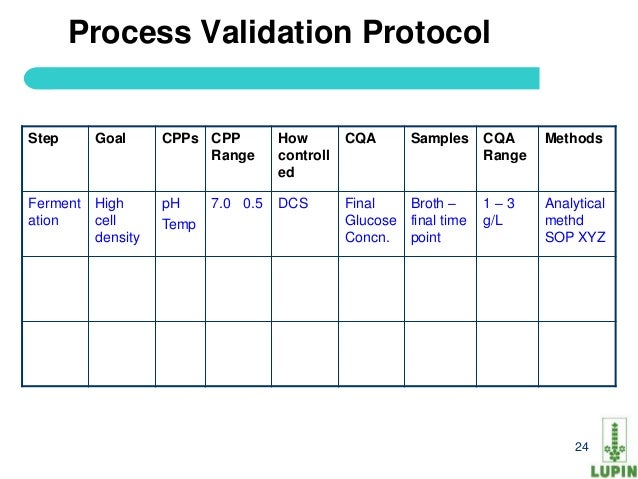
Process validation protocol template for api. Process validation protocol reference. Process parameters unrelated to quality such as variables controlled to minimize energy consumption or equipment use need not be included in the process validation. Powerful process validation app to ensure product quality and compliance with fda regulations. Process validation should confirm that the impurity profile for each api is within the limits specified.
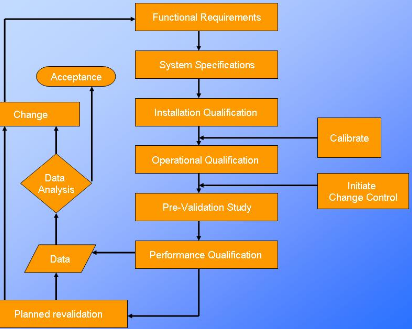
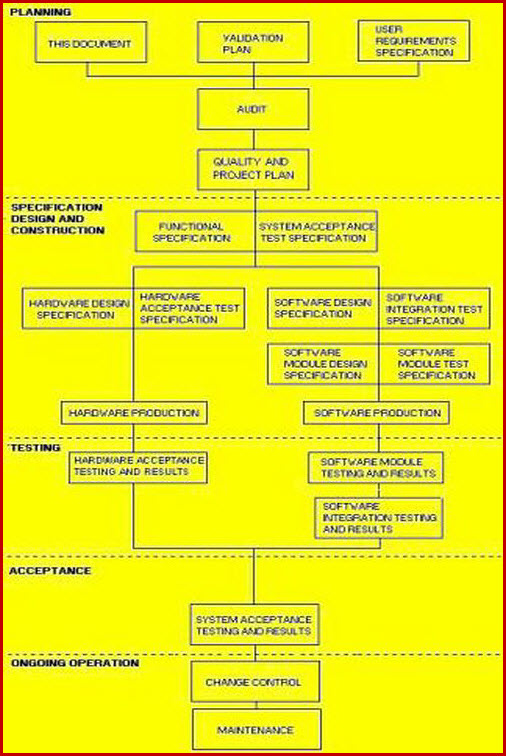
This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological products. For process validation the protocol would identify the number of validation batches. Process validation deviations deviations from the signed and approved methodology procedure or expected versus actual results will be recorded on the deviation log and summary form in appendix 7 and categorized as critical and non critical. Process validation sample protocol process validation protocol template or format for the products manufactured in the pharmaceutical product manufacturing facility.
Here are the details of validation protocol report format types pdf ppt. Sop page 14 of 24 10. 1 process validation report template and process validation protocol templates for 2 equipment qualification 3 installation qualification 4 operational qualification and 5 performance qualification. Process validation template types format pdf.
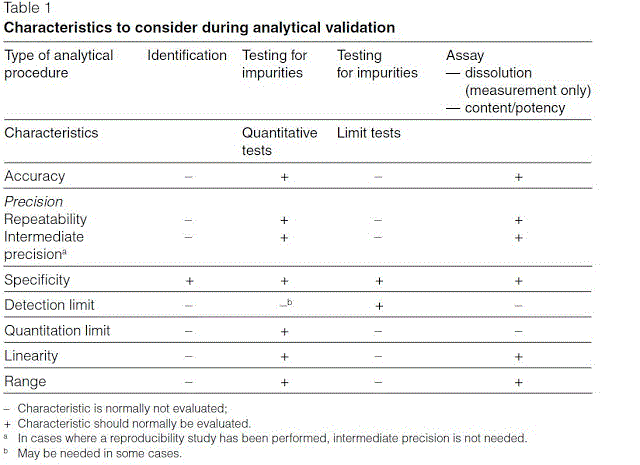
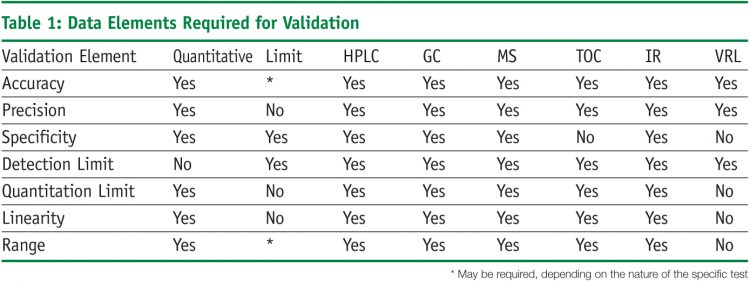
33 api starting material a raw material intermediate or an api that is used in the production of an api. Study this protocol was generated and approved to validate a high performance liquid chromatographic hplc stability indicating method for the analysis of compound a and its impurities related a and related b in your product 5 and 10 mg tablets. Quality safety and efficacy are tested along wth in process and finished product inspection or testingpharmaceutical process validation protocol report format example ppt pdf. Analytical validation seeks to demonstrate that the analytical methods yield results which permit an objective evaluation of the quality of the pharmaceutical product as specified.
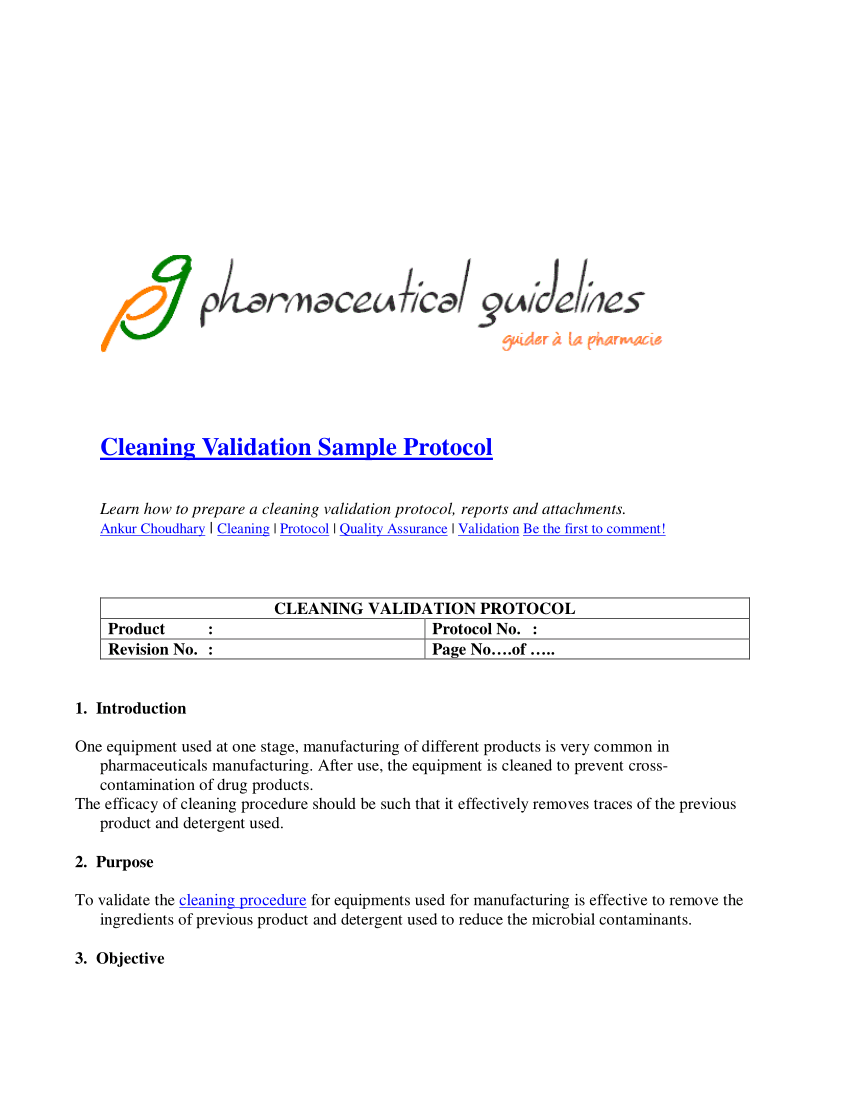
Process validation protocol pharmaceutical template pdf ppt xls this is to assure drug quality. 5 of the best process validation report templates.