Pharmacovigilance Certificate Course

Our course provides practical experience of using the oracle argus software and other techniques imperative for a career in pharmacovigilance.
Pharmacovigilance certificate course. The courses take a microlearning approach where each lesson is short concise and connected to a clear learning objective. Our modular pharmacovigilance training is recognized worldwide. By using our pharmacovigilance online courses you can develop your skills to help meet the growing global need for pharmacovigilance. Introduction to collecting and reporting adverse events this short course provides a general introduction and overview of adverse events and how to deal with them when they occur.
Qualified pharmacovigilance associate certification pviacr frequently asked questions is it compulsory to do an iaocr training course. It is consistent with the legislation changes in eu and is suitable for everyone who needs to be up to date with the pharmacovigilance guidelines or is involved in drug safety pharmacovigilance regulatory and quality compliance. Our free gvp training can also serve as a refresher course. In addition to their duty to protect public health in.
In response to the global need for training in pharmacovigilance umc has developed free online courses covering basic pharmacovigilance signal detection and causality assessment and statistical reasoning. The accreditation process is focused on determining whether someone has the skills knowledge and behaviors to do their role. Pharmacovigilance related e learning courses available on the global health network. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with us and eu regulations.
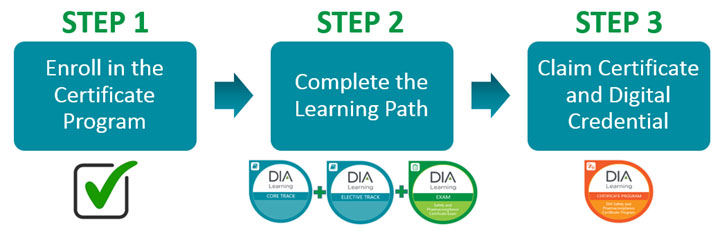
At sj pharma consulting llc in mendhamnj we provide drug safety and pharmacovigilance training courses that cover the us eu and ich regulatory requirements. All marketing authorization holders are now required to have internal pv audits of their pharmacovigilance operations. Dias safety and pharmacovigilance certificate program is a comprehensive program based on the dia safety and pharmacovigilance competency framework developed with experts working in the field. This newly updated course includes quality elearning delivery with voiceovers and illustrationsdrug safety monitoring and risk management are vitally important for medicinal product developers license holders and clinical investigators.